How to Calculate Ph
Where pH is then calculated with pH log 10 H 15 The root of Eq. How do you calculate the H concentration.

Trick To Calculate The Ph From Molarity And Normality Youtube Physical Chemistry How To Find Out Net Exam
Enjoy low prices on earths biggest selection of books electronics home apparel more.

. To calculate it take the log of a given hydrogen ion concentration and reverse the. PH Formula is expressed as. We know the formula for calculating the pH when you are given molarity is.
Go through the simple and easy guidelines on how to measure pH value. PH Formula Related Problems. Ad Manufactured Since 1934 Our pH Tests Set The Standard For Quality Accuracy.
How to Calculate pH. The pH is then calculated using the expression. PH is the negative logarithm of 10 log in the calculator of the hydrogen ion concentration of a solution.
The procedure to use the pH calculator is as follows. Ad Browse discover thousands of brands. Up to 24 cash back Plug in the information into the formula.
We Specialize in Manufacturing the most Trusted pH Paper and Saliva pH Test Kits. This video explains how to calculate the H ion given the p. The pH Formula can also be expressed as.
EqpH pKa logfracbaseacid eq Plug in values. EqpH 753 logfrac0502 eq Solve. Finally the pH value will be displayed in the new window.
Now click the button Calculate to get the pH value. The Newton-Raphson is the fastest but can sometimes diverge. PH - log H 3 O.
EqpH 793 eq. The pH of an aqueous solution can be determined and calculated using the concentration of hydronium ions in the solution. The user can choose bisection Newton-Raphson or Brents method Chapra and Canale 2006 Chapra 2007 as specified on the QUAL2K sheet.
PH -log 10 H Here H represents the value of molarity so when you have a value of H or molarity you can calculate the pH value by taking the logarithm to the base 10 of the H or molarity. Click on the Calculate button to obtain the pH value. Enter the name of the chemical solution and its concentration value in the respective input fields.
Know the concentration of hydrogen ions in the solution. The hydronium ion concentration is. How to calculate the pH for a specific temperature.
14 is determined with a numerical method. Find out the pH of the solution in which the concentration of hydronium ion is 80 10-8 M. Enter the chemical solution name and its concentration value in the respective input field.
POH 0699 14. In this case we are finding pOH and pH is known so the formula is. Calculating H from pH Acids Bases Tutorial.
Plug in the information into the formula. A neutral pH of 7 equates to a hydronium ion concentration of 10 -7 M. Find the pH of a 00025 M HCl solution.
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter molarity. Introduces and gives examples of pH calculation from the concentration of strong acids or bases. PH is the negative base 10 logarithm log on a calculator of the hydrogen ion concentration of a solution.
Pick one of the formulas. Calculate the pH by using the pH to H formula ie pH-log H You can also calculate the pOH and. Think about your answer.
Now what is the pOH of the solution above. The HCl is a strong acid and is 100 ionized in water. Read customer reviews find best sellers.
Copy the Henderson-Hasselbalch equation. Finally the pH value will be visible in a new window. To use the pH calculator follow these steps.
To calculate it take the logarithm of the given hydrogen ion concentration and reverse the sign. PH-log02M Enter and look on the graphing calculator for the answer.
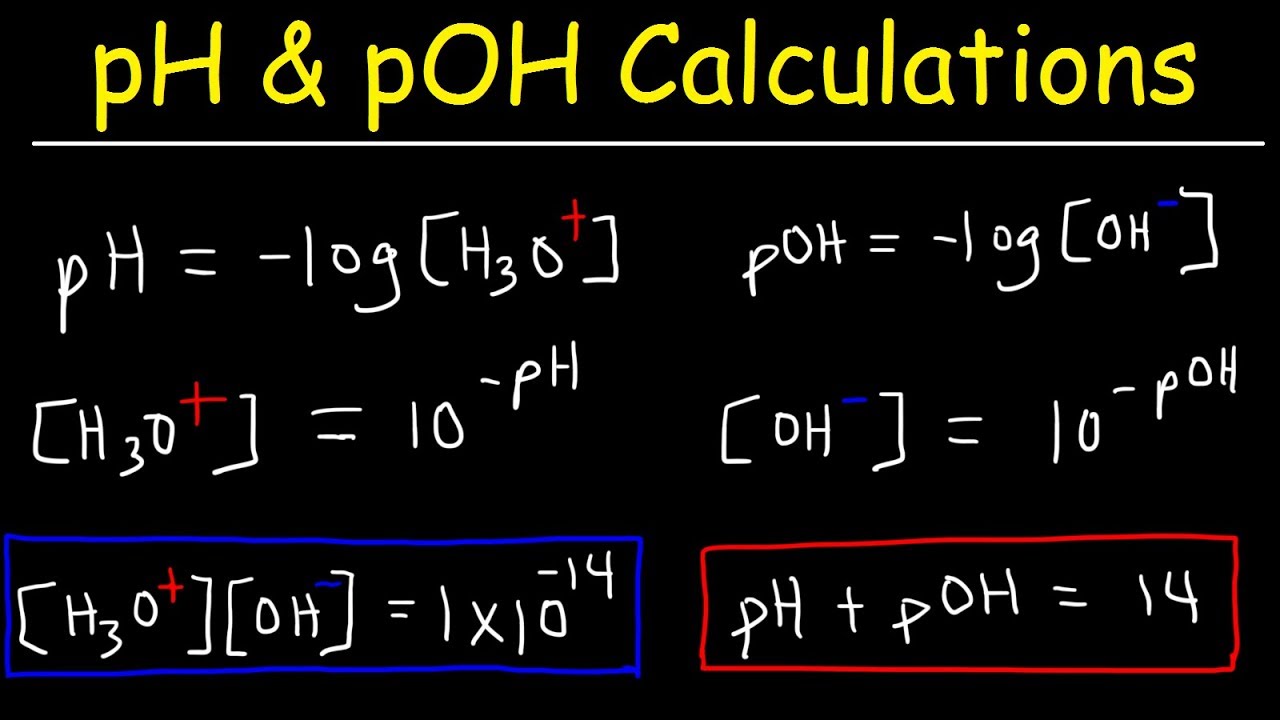
Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Teaching Chemistry Chemistry Lessons Chemistry Classroom

Ph Poh H3o Oh Kw Ka Kb Pka And Pkb Basic Calculations Acids And Bases Chemistry Problems Teaching Chemistry Chemistry Lessons Chemistry Classroom

How To Calculate Ph If Hydrogen Ion Concentration Is Given For All Stud Student Concentration Calculator

No comments for "How to Calculate Ph"
Post a Comment